Drug-induced alopecia - Hair and Scalp
Alerts and Notices
Important News & Links
Synopsis

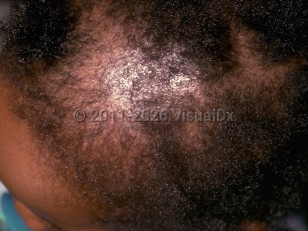
In most cases, drugs induce telogen effluvium. Common culprit medications include endocrine therapies such as selective estrogen receptor modulators, retinoids, anticoagulants, antithyroid drugs, lithium, anticonvulsants, beta blockers, immunosuppressive agents such as methotrexate and azathioprine, NSAIDs, levodopa, and allopurinol.
Cytotoxic drugs can cause anagen effluvium, leading to rapid hair loss within days to weeks of starting the offending medication.
Cancer treatment-induced alopecia depends on the specific agent and the route, dose, and schedule of administration. High-dose, intermittent, intravenous (IV) chemotherapy is more likely to cause higher-grade alopecia than low-dose therapy, oral administration, and weekly IV regimens. Combination therapies are more likely to cause some level of alopecia compared to single agents. Chemotherapy agents at highest risk of causing alopecia include cytotoxic agents (ie, alkylating agents, antitumor antibiotics, antimicrotubule agents, and topoisomerase inhibitors), endocrine therapy (ie, estrogen antagonists, aromatase inhibitors, and CDK4/6 inhibitors), and molecularly targeted agents (eg, EGFR inhibitors, hedgehog signaling pathway inhibitors). Other risk factors include poor drug metabolism, prior exposure to scalp irradiation, older age, presence of androgenic alopecia, prior chemotherapy-induced alopecia, and graft-versus-host disease.
Reversibility of chemotherapy-induced alopecia depends on the degree of damage to the hair follicle stem cells. Permanent chemotherapy-induced alopecia is associated with both taxane and busulfan chemotherapy agents, most notably from docetaxel. The pathophysiology is believed to be secondary to irreversible damage to the hair follicle, with the ABCB1 gene variation involved. Changes in the hair color or texture as well as only partial hair regrowth have been reported. The typical timeline clinically for chemotherapy-induced alopecia is incomplete or absent hair regrowth 6 months postchemotherapy.
Drug-induced alopecia areata is associated with tumor necrosis factor-alpha (TNF-α) inhibitors, dupilumab, and the immune checkpoint inhibitors.
Scarring alopecias, including drug-induced lichen planopilaris (LPP), folliculitis decalvans, and erosive pustular dermatosis of the scalp (EPDS), have been linked to tyrosine kinase inhibitors, PD-1 inhibitors, TNF-α inhibitors, and EGFR inhibitors.
Localized alopecia has been reported as an adverse effect in men at the site of deoxycholic acid injections for submental adiposity.
The most common cause of drug-induced alopecia in children is chemotherapy-induced anagen effluvium.
Thiotepa is the drug most commonly involved in permanent chemotherapy-induced alopecia.
Codes
L64.0 – Drug-induced androgenic alopecia
L65.9 – Nonscarring hair loss, unspecified
T50.905A – Adverse effect of unspecified drugs, medicaments and biological substances, initial encounter
SNOMEDCT:
73383004 – Drug-related alopecia
Look For
Subscription Required
Diagnostic Pearls
Subscription Required
Differential Diagnosis & Pitfalls

Subscription Required
Best Tests
Subscription Required
Management Pearls
Subscription Required
Therapy
Subscription Required
Drug Reaction Data
Subscription Required
References
Subscription Required
Last Updated:12/29/2025