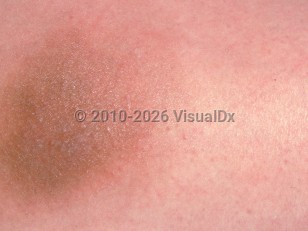
Vitamin K injection site reaction
Alerts and Notices
Important News & Links
Synopsis

Reactions are thought to be caused by a type IV delayed type hypersensitivity with two distinctive phenotypes: eczematous and sclerodermoid. One reported case of a diffuse rash has been attributed to vitamin K injection.
Eczematous reactions tend to appear within a week of injection and resolve over the course of a month. The reaction is localized to the general proximity of injection. Sclerodermoid changes can be seen several months after injection and persist for years despite treatment. Preceding inflammation may or may not be observed.
Previously, it was thought that patients with underlying liver disease who received phytonadione injection were at an elevated risk to develop injection site reactions. More recent review of existing case reports has found this not to be the case.
Patch testing to vitamin K has not been found to be beneficial in predicting this reaction, although intradermal prick testing has been positive in affected patients in one study.
Codes
L25.1 – Unspecified contact dermatitis due to drugs in contact with skin
SNOMEDCT:
860897009 – Injection site eruption
870413003 – Adverse reaction to vitamin K and vitamin K derivative
Look For
Subscription Required
Diagnostic Pearls
Subscription Required
Differential Diagnosis & Pitfalls

Subscription Required
Best Tests
Subscription Required
Management Pearls
Subscription Required
Therapy
Subscription Required
Drug Reaction Data
Subscription Required
References
Subscription Required